China’s financing and investment spread across 61 BRI countries in 2023 (up...
2024-02-27 31 英文报告下载
The US CDC has started using its flu surveillance network to screen for coronavirus. In particular, at the 15 top volume labs if a sample comes in and tests negative for influenza A/B then the lab run coronavirus testing. Over the next few week, an increase in US cases (or a lack of any new cases) from this effort could impact perception. • The international team from the WHO (including experts from US) arrived in China this week. We would expect views from that team over the next 1-2 weeks. Their conclusions in terms of severity and ability to contain the virus will be important drivers of investor’s perception. • We expect the Moderna coronavirus vaccine to enter clinical testing in people in mid-March/early April, based on comments from the US NIH. It takes a month to determine if patients have appropriate protection from the vaccine, so the earliest initial results we could expect will be ~2-3 months after the first group of patients are dosed.
Gilead’s remdesivir is being tested in two clinical studies in China. This is to treat patients already with the disease. Final results are expected in early April, but we could get some interim data by end of Feb/early March. On 2/15, the State Council of China announced the progress of the trials. By the time of announcement, 168 patients have been enrolled to the trial of severe disease, and 17 patients to the trial of mild/moderate disease. However, progress has been reportedly slowed down by a lack of eligible recruits as they must not have received other treatment in the last 30 days. We could expect the results from the trial of severe disease in late March, but the results from the mild/moderate disease trial may come out later than expected.
There are a handful of studies using repurposed HIV drugs in patients in China. Those datasets should start to come out in March/April timeframe. • Nan-shan Zhong, the leading advisor of the national crisis management team, published a preprint paper on 2/9. In the study of 1,099 patients with laboratory-confirmed COVID-19, the median incubation period was estimated to be 3.0 days (range: 0 to 24.0 days). The median is on the low end of previous estimates, but the upper limit is more than 14 days, which is the current recommended quarantine period. This means a small portion of patients could have long incubation period, which is consistent with the asymptomatic infections observed in several areas, including on the Diamond Princess cruise ship. However, this does not mean patients with asymptomatic infections could spread the disease, but it is a sign of the needs for more strict quarantine measures.

标签: 英文报告下载
相关文章
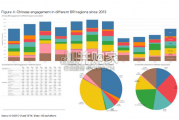
China’s financing and investment spread across 61 BRI countries in 2023 (up...
2024-02-27 31 英文报告下载

Though the risk of AI leading to catastrophe or human extinction had...
2024-02-26 52 英文报告下载
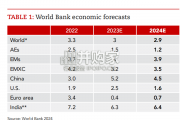
Focusing on the prospects for 2024, global growth is likely to come i...
2024-02-21 96 英文报告下载

Economic activity declined slightly on average, employment was roughly flat...
2024-02-07 67 英文报告下载
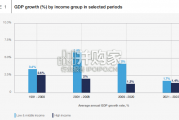
Economic growth can be defned as an increase in the quantity or quali...
2024-02-06 82 英文报告下载
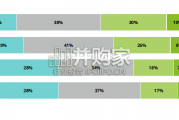
In this initial quarterly survey, 41% of leaders reported their organizatio...
2024-02-05 66 英文报告下载
最新留言